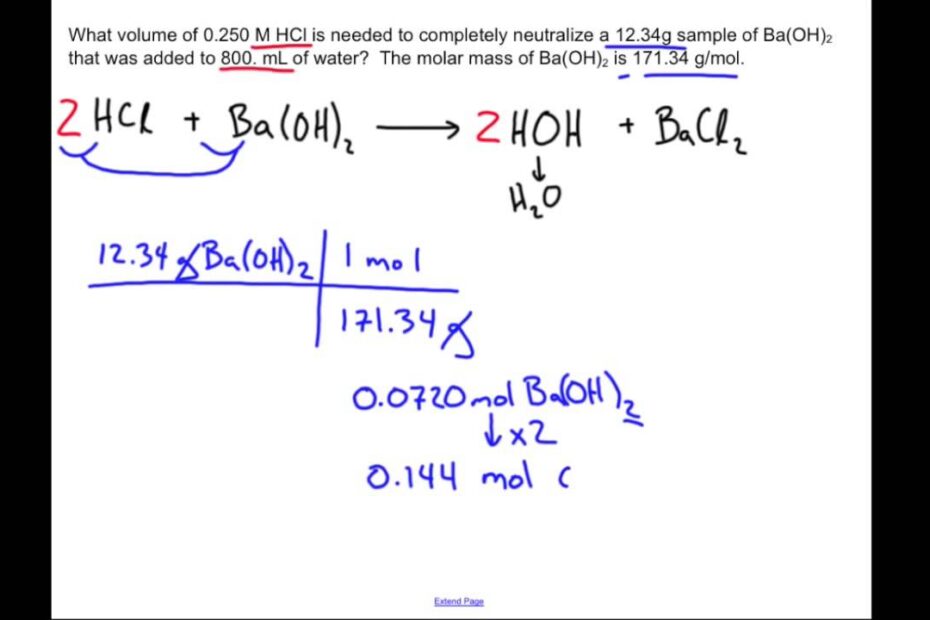
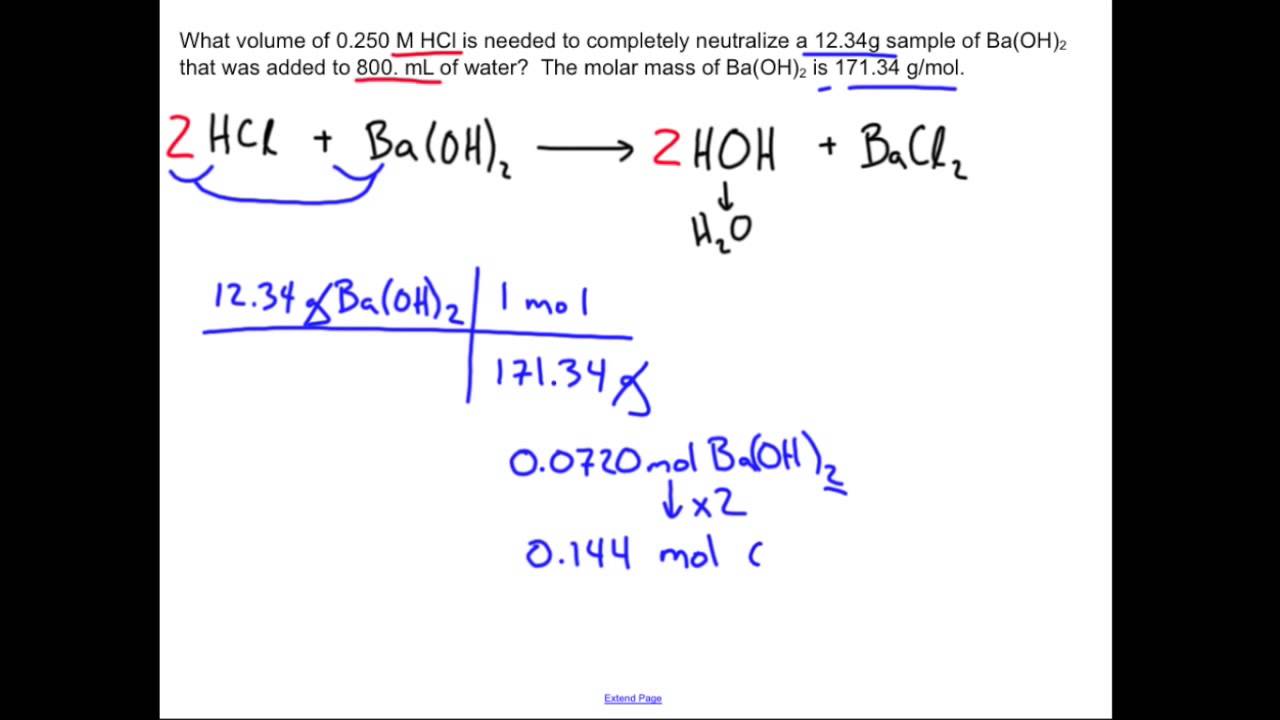
Unlocking The Mystery: How Do You Calculate The Neutralization Of A Mole?
Neutralization Reaction: Chemistry Sample Problem
Keywords searched by users: How do you calculate the neutralization of a mole moles neutralized calculator, titration neutralization formula, volume needed to neutralize calculator, neutralization formula calculator, neutralizing an acid with a base calculations, how to find moles of oh reacted, neutralization titration definition, molarity neutralization formula
What Is The Formula For Neutralization?
Neutralization reactions occur when an acid and a base react, resulting in the formation of water and a salt, which is a neutral ionic compound. To illustrate this process, let’s examine the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) solutions. The balanced chemical equation for this neutralization reaction is:
HCl + NaOH → H2O + NaCl.
In this reaction, hydrochloric acid and sodium hydroxide combine to produce water (H2O) and sodium chloride (NaCl). This reaction is a fundamental example of how acids and bases interact to form neutral products.
What Is The Formula For Molarity Of Neutralization?
The formula for calculating the molarity of neutralization involves finding the product of molarity and volume. Specifically, you can determine the molarity of protons by multiplying the molarity of hydroxide ions by their respective volume. This equation is based on the fundamental relationship that moles are equal to molarity times volume. This approach is commonly employed when determining the molarity of acids after a neutralization reaction. For a more detailed explanation, you can refer to educational resources available on platforms like YouTube.
What Is Neutralisation In Mole Concept?
Neutralization in the context of the mole concept refers to a chemical reaction where hydrogen ions (H+) and hydroxide ions (OH-) combine to completely neutralize each other. This neutralization reaction occurs when the number of H+ ions is equal to the number of OH- ions. To provide a clearer understanding, for every 1 mole of hydroxide ions, you need precisely 1 mole of hydrogen ions to achieve complete neutralization. This balanced combination ensures that the resulting solution is neither acidic nor basic, but instead, it attains a neutral pH. In essence, neutralization reactions are fundamental in chemistry for regulating pH levels and understanding the stoichiometry of these reactions is crucial in chemical calculations.
Found 19 How do you calculate the neutralization of a mole




Categories: Summary 49 How Do You Calculate The Neutralization Of A Mole
See more here: future-user.com

Learn more about the topic How do you calculate the neutralization of a mole.
- Neutralization Reaction | CK-12 Foundation
- Finding molarity of acids after neutralization – YouTube
- Neutralization Reactions Chemistry Questions with Solutions – BYJU’S
- 21.4 Acid Base Neutralization – CK-12
- Definition, Neutralization Reaction, Applications. – BYJU’S
- Neutralization number Definition & Meaning – Merriam-Webster
See more: https://rausachgiasi.com/your-money blog