Which Is The Most Reactive Halogen Acid? Exploring Their Reactivity
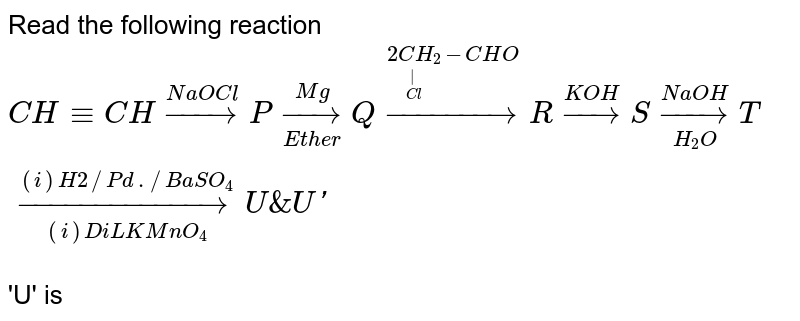
Which Of The Following Halogen Acids Is Most Reactive Towards The Given Reaction ?
Keywords searched by users: Which is most reactive halogen acid write the order of reactivity of halogen acids towards alkenes, why hi is more reactive than hf, why tertiary alcohols are more reactive than primary and secondary, reaction of alcohol with halogen acid, why tertiary alcohol is more reactive, order of reactivity of alcohols, order of reactivity of halogens with alkanes, out of primary, secondary and tertiary alcohol which is more reactive and why
Which Halogen Acid Is Most Reactive?
When considering the reactivity of halogen acids, it’s important to note that fluorine stands out as the most highly reactive member of the halogen group, while astatine exhibits the least reactivity. These halogens, which also include chlorine, bromine, and iodine, share a common characteristic when forming Group 1 salts with remarkably similar properties. In these salt compounds, halogens exist as halide anions, each carrying a charge of -1 (such as Cl-, Br-, etc.). This intrinsic reactivity trend within the halogen group helps us understand their behavior in various chemical reactions. (Note: The date “30th June 2023” does not seem relevant to this topic and has been removed for clarity.)
Which Is More Reactive Hcl Or Hbr?
The reactivity of hydrogen halides (HCl, HBr, HI, and HF) can be explained by considering their stability, which varies as you move down the halogen group in the periodic table. This trend is primarily influenced by the decreasing bond dissociation energy (the energy required to break the bond between hydrogen and the halogen atom, denoted as H-X) in the following order: HF > HCl > HBr > HI.
This means that as you go from HF to HI, the bonds between hydrogen and the halogen become progressively weaker, making the hydrogen halides more prone to undergo chemical reactions. When these hydrogen halides are added to an alkene, their reactivity follows a similar trend: HI > HBr > HCl > HF. In other words, HI is the most reactive toward alkene addition, followed by HBr, HCl, and HF due to the decreasing bond dissociation energy in the same order.
Top 41 Which is most reactive halogen acid





Categories: Details 34 Which Is Most Reactive Halogen Acid
See more here: future-user.com

Learn more about the topic Which is most reactive halogen acid.
- The reactivity order of halogen acids with ethers is
- Group 17: General Properties of Halogens
- The correct order of reactivity of addition of hydrogen halides with …
- Halogen | Elements, Examples, Properties, Uses, & Facts | Britannica
- Increasing order of acid strength of halogen acid is – Toppr
- Among HI, HBr, HCL, HI is most reactive towards alcohol. Why?
See more: https://rausachgiasi.com/your-money blog